Non-Steroidal Anti-inflammatory Drugs (NSAIDs) including Cyclo-oxygenase-2 (COX-2) Selective Inhibitors
Standard Non-Steroidal Anti-inflammatory Drugs (NSAIDs): Aspirin [analgesic/high-dose > 150mg], Dexibuprofen, Dexketoprofen, Diclofenac, Etodolac, Flurbiprofen, Ibuprofen, Indometacin, Ketoprofen, Mefenamic acid, Meloxicam, Nabumetone, Naproxen, Piroxicam, Sulindac, Tenoxicam, Tiaprofenic acid, Tolfenamic acid COX-2 Selective Inhibitors: Celecoxib, Etoricoxib |
Issues for Surgery |
Loss of pain control if omitted. For gout, inflammatory conditions, migraine, menorrhagia – loss of symptom control if omitted, which may impede post-operative recovery. For standard NSAIDs – increased risk of bleeding if continued. |
Advice in the Perioperative period |
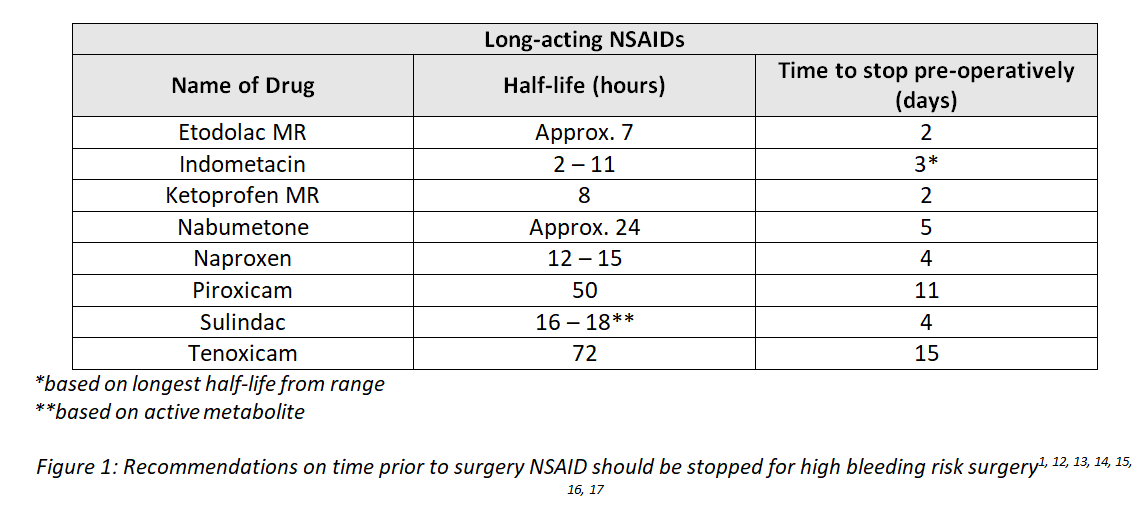
Elective Surgery Low Bleeding Risk Procedures Standard NSAIDs and COX-2 selective inhibitors: Continue1 – including combination products. EXCEPT: Aspirin – consider stopping 7 days pre-operatively, and using alternative analgesia, to minimise the risk of bleeding (including combination products). High Bleeding Risk Procedures Standard NSAIDs: The risk benefit of stopping NSAIDs pre-operatively should be considered, weighing up potential loss of pain control versus risk of bleeding. Except for aspirin, it may be beneficial to continue NSAID use until the day of surgery, ensuring that the Surgeon and Anaesthetist are aware of continued use. If the decision is made to stop NSAID pre-operatively, the optimal time for cessation is detailed below. Standard NSAIDs: -
NSAID Combination Products: -
If necessary, consideration should be given to prescribing the components of combination products as separate medicines perioperatively (particularly for the combination product dexketoprofen + tramadol). Analgesic Aspirin Combination Products: - (NB: there are many different over-the-counter [OTC] analgesic preparations that contain aspirin together with other medicines)
Short-acting NSAIDs*: Aceclofenac, Dexketoprofen, Diclofenac (modified and standard release preparations), Flurbiprofen, Ibuprofen (modified and standard release preparations), Mefenamic acid, Tiaprofenic acid, Tolfenamic acid2, 3, 4, 5, 6, 7, 8, 9, 10, 11. * based on half-life < 4.8 hours
Emergency Surgery For high-risk bleeding procedure: If there is insufficient time to follow the advice above be aware of the potential for increased bleeding if patient has taken doses in the days leading up to surgery. Perioperative Considerations Neuraxial Anaesthesia (see Further Information) Post-operative Advice NSAIDs are a valuable option for post-operative pain relief in those patients in whom their use is not contraindicated. They may be useful alternatives or adjuncts to opioids19; however, in surgery with high risk of bleeding or where bleeding can result in catastrophic outcome, such as ophthalmic or neurosurgery, the decision to prescribe NSAIDs should be made on a case-by-case basis. Furthermore, NSAIDs may be associated with increased risk of gastrointestinal (GI) anastomotic leak and caution is recommended when using NSAIDs after GI surgery4. An intravenous NSAID should not be used to manage immediate post-operative pain (including pain after dental surgery) unless the patient cannot take oral medicines20. Monitor renal function – there is an increased risk of acute kidney injury (AKI) in patients with use of NSAIDs after surgery (especially in patients undergoing emergency surgery or intraperitoneal surgery)21. NSAIDs may mask fever and other signs of inflammation or infection3, 4, 6, 7, 12, 17, 19, 22, 23 – close monitoring for post-operative infection is advised. NSAIDs should be prescribed at the lowest effective dose and for the shortest duration possible to minimise adverse effects, particularly in those at increased risk of serious adverse reactions (e.g. elderly, impaired renal, cardiovascular or hepatic function)2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 19, 23, 24, 25, 26, 27, 28. If the surgery has addressed the cause of the pain, NSAID analgesia should be weaned post-operatively with a view to stopping completely. |
Interaction(s) with Common Anaesthetic Agents |
Thiopental The anaesthetic dose of thiopental is reduced and its effects prolonged in patients who have been pre-treated with aspirin (at analgesic dose)29. Bupivacaine One study suggests that the failure rate of spinal anaesthesia with bupivacaine is markedly increased in patients receiving indometacin. The clinical significance of this interaction is unclear but should be borne in mind in case of unexpected response to treatment29. Benzodiazepines Diazepam might increase diclofenac exposure and have a small effect on the pharmacokinetics of ibuprofen, although the clinical significance of this interaction is unclear29. Additive dizziness might occur with diazepam and indometacin, bear this in mind should an increase in dizziness occur29. Diclofenac moderately reduces both sedative and hypnotic doses of intravenous midazolam; bear the interaction in mind in case of an unexpected response to treatment29. |
Interaction(s) with other Common Medicines used in the Perioperative Period |
Multiple NSAID Use Multiple NSAID use should be avoided2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 22, 23, 25, 26, 27, 28. Ketorolac is an NSAID that is often used as an adjunctive pain relief perioperatively; hence there is an increased risk of NSAID related adverse effects if ketorolac is used concomitantly with another NSAID22. Bleeding Risk Low molecular weight heparin (LMWH), unfractionated heparin (UFH) and oral anticoagulants (warfarin, direct oral anticoagulants [DOACs]) are predicted to increase the risk of bleeding events when given with NSAIDs2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 19, 23, 25, 26, 27, 28, 29, 30, 31 (see also Hyperkalaemia and Hyponatraemia below). Gastrointestinal Ulceration and Bleeding Corticosteroids may increase the incidence and / or severity of ulceration associated with NSAIDs, and increases the possibility of gastrointestinal bleeding2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 19, 22, 24, 25, 26, 27, 28. Caution with concomitant use and consider the use of gastroprotection such as histamine-2 receptor antagonist or proton pump inhibitor2, 3, 4, 6, 7, 9, 10, 11, 12, 13, 14, 15, 16, 17, 23, 24, 25, 27. This interaction is unlikely to be an issue where corticosteroids are used as single doses to reduce post-operative nausea and vomiting or as cover for patients at risk of adrenal insufficiency. Nephrotoxicity There is an increase in the risk of nephrotoxicity if NSAIDs are used concurrently with the following antimicrobials3, 10, 19, 29: -
Hyperkalaemia and Hyponatraemia Concomitant use of NSAIDs with the following medications can increase the risk of hyperkalaemia4, 19, 22: -
Concomitant use of NSAIDs and trimethoprim can increase the risk of hyponatraemia19. Antimicrobials NSAIDs potentially increase the risk of seizures when given with quinolones (e.g. ciprofloxacin)2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 19, 25, 27. Seizures are rare, so in most patients concurrent use should be without problem. Avoid concurrent use or monitor closely those patients with epilepsy or who are predisposed to seizures29; however, due to the increased risk of seizure associated with quinolones, they are generally avoided in those who are predisposed to seizures. NSAIDs may reduce excretion of aminoglycosides (e.g. gentamicin)7, 10, 17, 19, 22, which may lead to accumulation and increased risk of adverse effects (also see Nephrotoxicity above). NSAIDs may reduce excretion of daptomycin – monitor the patient for possible daptomycin adverse effects 29. NSAIDs are highly protein bound, hence concomitant administration with other highly protein-bound drugs such as sulphonamide antibiotics (e.g. co-trimoxazole) should be done with caution and overdose symptoms carefully monitored16, 17 (also see Hyperkalaemia and Hyponatraemia above). Metoclopramide Metoclopramide modestly reduces the bioavailability of ketoprofen due to its prokinetic effects. The relatively poor solubility of ketoprofen means that it spends less time in the stomach where it dissolves, and as a result less is available for absorption in the small intestine. The clinical importance of this is unknown, but the recommendation is that ketoprofen (and possibly other NSAIDs that are poorly soluble) should be taken 1 – 2 hours before metoclopramide29. Conversely, for other NSAIDs (i.e. aspirin, tolfenamic acid), metoclopramide can be used to increase the rate of absorption, and may possibly speed up the onset of analgesic effect29. |
Further Information |
COX-1 vs. COX-2 Inhibition and Bleeding Risk The COX enzymes are responsible for the production of prostaglandins. There are 2 types of COX enzymes – COX-1 and COX-2. Both enzymes produce prostaglandins that promote inflammation, pain and fever; however, only COX-1 produces prostaglandins that activate platelets and protect stomach and intestinal lining. Aspirin irreversibly affects COX-1, leading to inhibition of platelet aggregation and vasoconstriction for the entire life cycle of the platelet. Standard NSAIDs (e.g. ibuprofen) reversibly inhibit the COX enzyme. NSAIDs vary in their selectivity for inhibiting COX-1 and COX-224 and thus their effects on platelet activity and they should not be compared to aspirin1. Due to their reversible action, the bleeding risk is reduced on cessation of therapy (see Evidence Base for Discontinuing NSAIDs Pre-operatively). COX-2 selective inhibitors (e.g. celecoxib) have less effect on platelet activity due to their selectivity for the COX-2 enzyme. They are not associated with increased bleeding risk in the perioperative period and are safe to continue for both elective and emergency surgery1 although consider possible interactions as detailed in Interaction(s) with Common Anaesthetic Agents and Interaction(s) with other Common Medicines used in the Perioperative Period). Although not labelled as COX-2 selective inhibitors, both meloxicam and etodolac are more COX-2 selective than celecoxib. Meloxicam’s ratio of COX-2:COX-1 inhibition is approximately 80:25, and it has not been found to have significant effects on platelets or increased bleeding risk1. Bear in mind that COX-2 selective inhibitors should not be prescribed in patients with concurrent cardiovascular disease due to increased risk of MI and stroke19. Evidence Base for Discontinuing NSAIDs Pre-operatively Recommendations on when to stop NSAIDs pre-operatively can sometimes be without the appropriate evidence-base. Discontinuation of NSAIDs should be based on the pharmacokinetics of the drug, COX selectively, patient factors (i.e. renal/hepatic function, pain level and tolerability) and the overall bleeding risk associated with the surgery. In general, levels of active drug reach an acceptably low level within 5 half-lives of stopping therapy1. Renal Toxicity Adequate fluid intake should be ensured during treatment with NSAIDs to prevent dehydration and possible associated increased renal toxicity24. Caution should be taken when initiating treatment with NSAIDs in patients with considerable dehydration3, 6, 7, 9, 26. Hypertension Due to inhibition of prostaglandin synthesis, NSAIDs can cause fluid retention, oedema, and hypertension2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 25, 26, 27, 28. Etoricoxib may be associated with more frequent and severe hypertension than some other NSAIDs and selective COX-2 inhibitors and the manufacturer specifically recommends that blood pressure (BP) should be checked prior to initiation of etoricoxib, within 2 weeks of initiation and periodically thereafter26. BP should be routinely monitored with all other NSAIDs. Regional Anaesthesia NSAIDs, when used alone, are not a contraindication to regional anaesthesia18, 32. However, consideration should be given to patients receiving other agents, which may increase the risk of bleeding (e.g. LMWH, aspirin), if regional anaesthesia is planned32.
|
References |
|